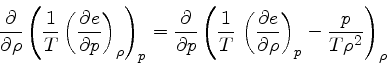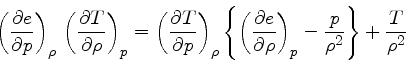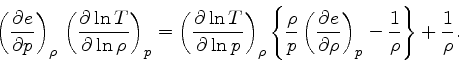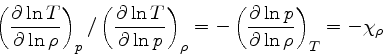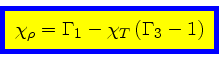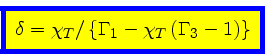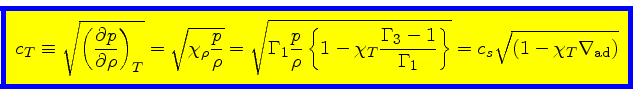



Next: 2.3.5 Ideal gas with
Up: 2.3 A collection of
Previous: 2.3.3 CO5BOLD equation of
Contents
Index
First, the missing derivative
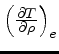 can be found from the relation:
can be found from the relation:
 |
(34) |
which is obtained from the equality of the mixed derivatives in
Eq.(16), written as:
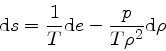 |
(35) |
Then
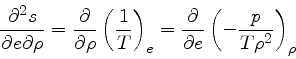 |
(36) |
First adiabatic exponent:
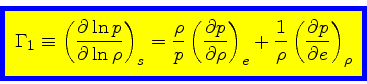 |
(37) |
This relation is obtained by combining Eq.(16) with the identity
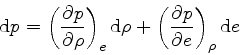 |
(38) |
The adiabatic sound speed
is then obtained as
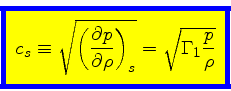 |
(39) |
Third adiabatic exponent:
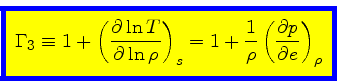 |
(40) |
This relation is obtained by combining Eq.(16) with the identity
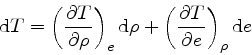 |
(41) |
and then using Eq.(34).
Adiabatic temperature gradient:
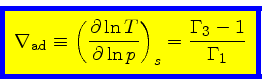 |
(42) |
since
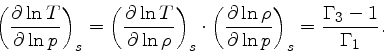 |
(43) |
Adiabatic energy changes:
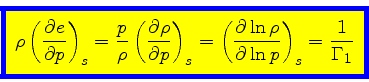 |
(44) |
or
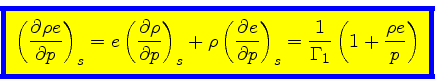 |
(45) |
We define the coefficients 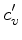 and
and 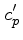 through the
relation
through the
relation
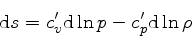 |
(46) |
Entropy change at constant density:
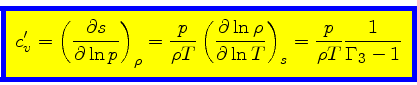 |
(47) |
This relation is obtained from the equality of the mixed derivatives in
Eq.(16) together with Eq.(40).
Entropy change at constant pressure:
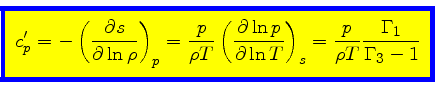 |
(48) |
This relation is obtained from the equality of the mixed derivatives in
Eq.(17) together with Eq.(42).
Specific heat at constant density:
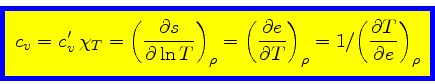 |
(49) |
To derive the specific heat at constant pressure, we start from the relation
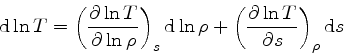 |
(50) |
from which we get
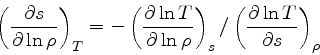 |
(51) |
Using Eqs.(40) and (49), we obtain
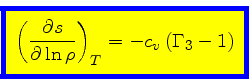 |
(52) |
Now
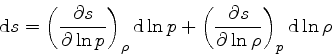 |
(53) |
or
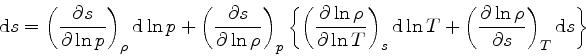 |
(54) |
hence
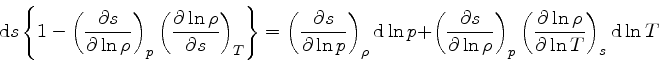 |
(55) |
and finally
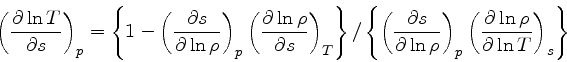 |
(56) |
or
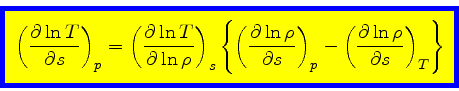 |
(57) |
Using Eqs.(23), (40), (48), (52),
we finally obtain the relation for the specific heat at constant pressure:
 |
(58) |
Alternatively, 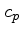 can be obtained from Eq.(28)
can be obtained from Eq.(28)
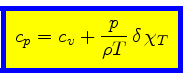 |
(59) |
or from
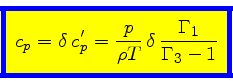 |
(60) |
once 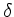 and
and  are known (see below).
are known (see below).
We can now express the thermodynamic coefficients provided by CO5BOLD
in terms of 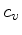 ,
, 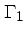 ,
, 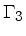 , and
, and
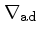 :
:
 |
(61) |
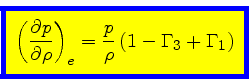 |
(62) |
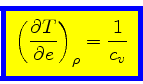 |
(63) |
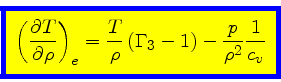 |
(64) |
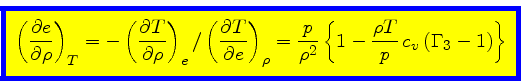 |
(65) |
 |
(66) |
We consider again Eq.(35), replacing 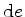 by
by
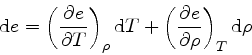 |
(67) |
so
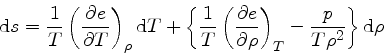 |
(68) |
The requirement that the mixed derivatives must be equal then yields
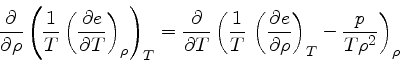 |
(69) |
or
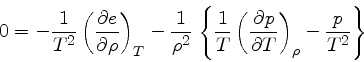 |
(70) |
Finally,
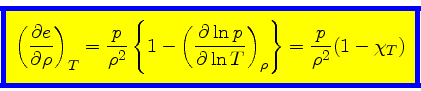 |
(71) |
Comparison with Eq.(65) implies
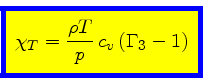 |
(72) |
Similarly, replacing 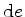 by
by
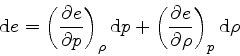 |
(73) |
in Eq.(35), we get
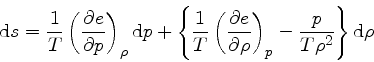 |
(74) |
and the requirement that the mixed derivatives must be equal then yields
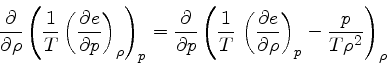 |
(75) |
or
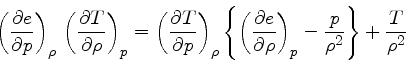 |
(76) |
or
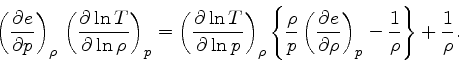 |
(77) |
Since
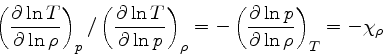 |
(78) |
we finally obtain, using Eqs.(25), (61) and (66),
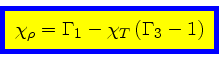 |
(79) |
and
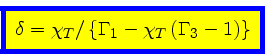 |
(80) |
The isothermal sound speed
is then obtained as
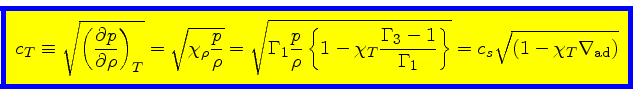 |
(81) |




Next: 2.3.5 Ideal gas with
Up: 2.3 A collection of
Previous: 2.3.3 CO5BOLD equation of
Contents
Index

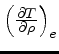 can be found from the relation:
can be found from the relation:
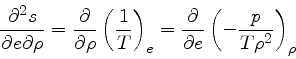
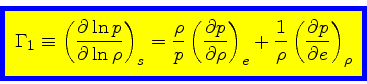
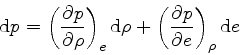
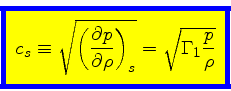
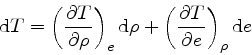
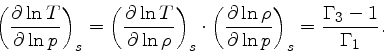
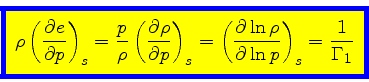
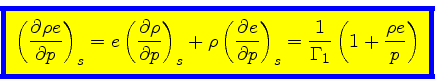
![]() and
and ![]() through the
relation
through the
relation
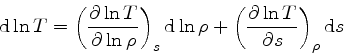
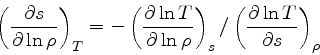
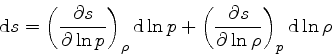
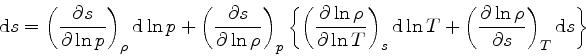
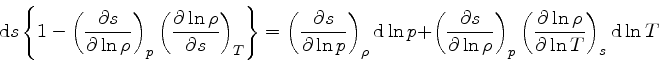
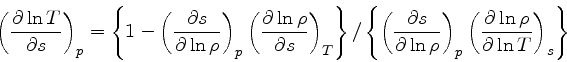
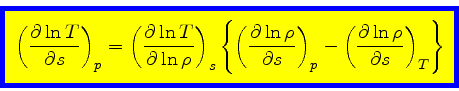

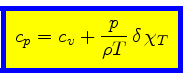
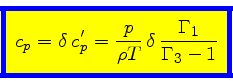
![]() ,
, ![]() ,
, ![]() , and
, and
![]() :
:
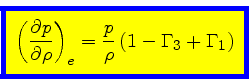
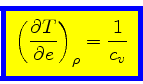
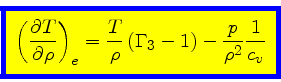
![]() by
by
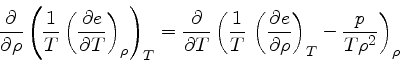
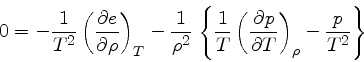
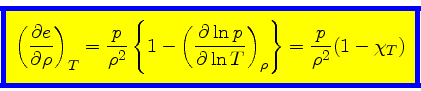
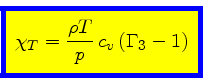
![]() by
by