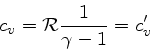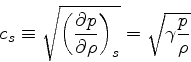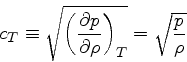



Next: 3 Program Files, Installation,
Up: 2.3 A collection of
Previous: 2.3.4 Derived thermodynamic coefficients
Contents
Index
2.3.5 Ideal gas with constant specific heats
(polytropic gas)
In this case, we obtain much simpler relations:
 |
(83) |
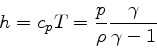 |
(84) |
 |
(85) |
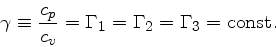 |
(86) |
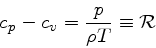 |
(87) |
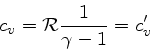 |
(88) |
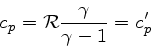 |
(89) |
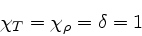 |
(90) |
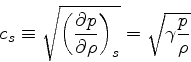 |
(91) |
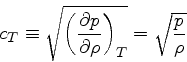 |
(92) |




Next: 3 Program Files, Installation,
Up: 2.3 A collection of
Previous: 2.3.4 Derived thermodynamic coefficients
Contents
Index